Long Measles: It's like Long Covid, But Deadlier.
How the measles virus can erode the brain and leave lasting neurological disabilities.
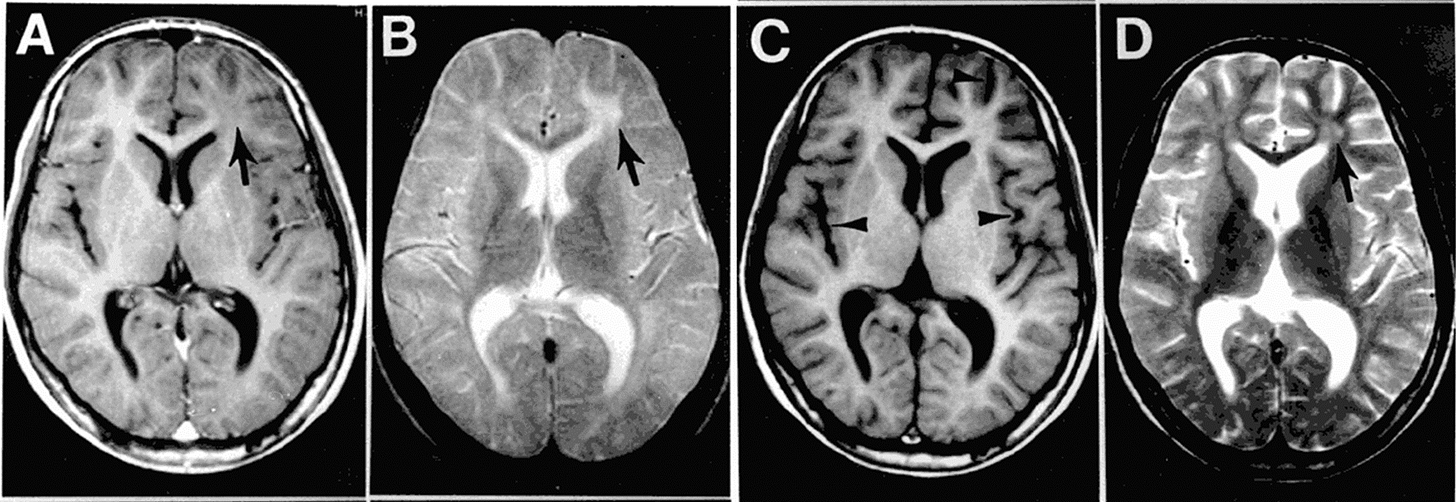
The above MRI scan belonged to a 13-year-old boy adopted from a Thai orphanage as a toddler, who had been thriving in Iowa with no known history of serious illness (Figure 1). But nearly a decade after arriving in the U.S., his behavior began to change. Teachers noticed memory lapses. His mother saw mood swings and tremors. Then came the seizures. By the time an MRI revealed widespread brain damage, it was too late. He had developed subacute sclerosing panencephalitis (SSPE), a rare brain neurodegenerative disease caused by a dormant measles virus. The boy did not survive. Tragically, his story is not as rare as most might think.
Most people think of measles as a childhood rash that comes and goes. But not many realize it’s actually a virus with the power to erase immune memory, hijack neurons, and resurface years later to incapacitate the brain. In the age of Long Covid, it’s time we talk about Long Measles.
Backdrop
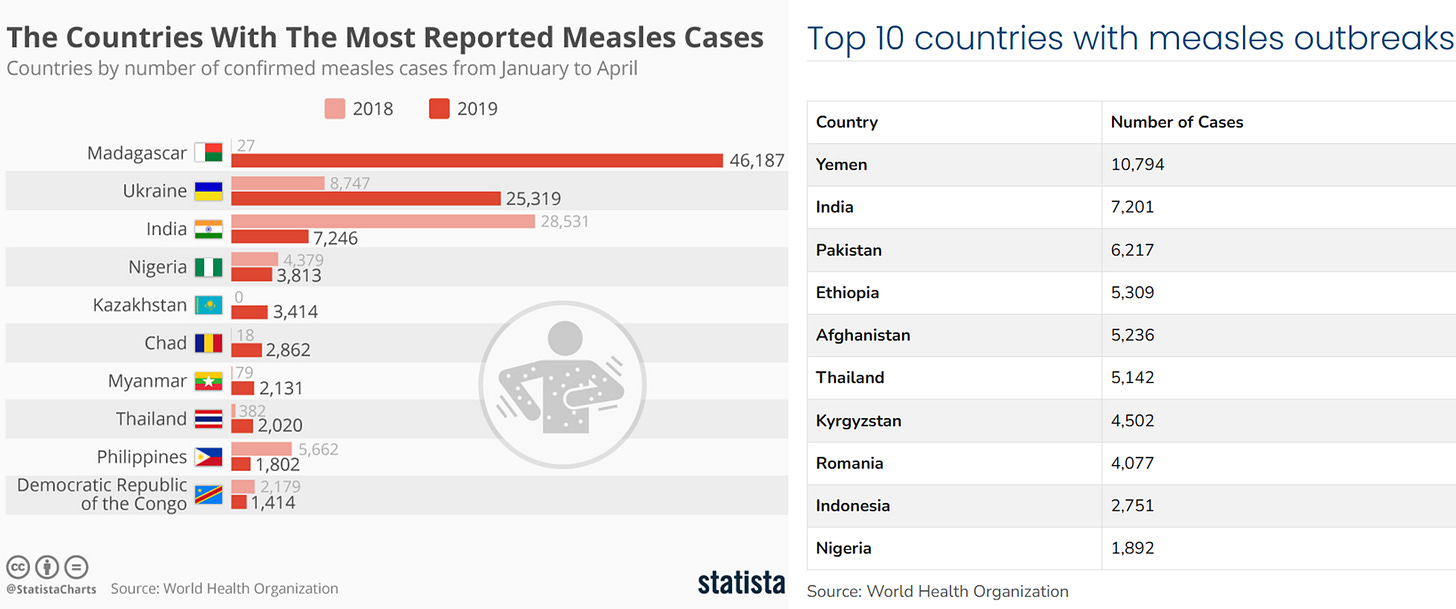
As an ancient infectious agent, the measles virus has resurfaced due to a drop in vaccination. In the U.S., over 700 confirmed measles cases had been reported as of April 10, 2025, already surpassing the 285 total cases reported in the previous year. Globally, over 127,000 measles cases were reported in over 50 countries in Europe and Central Asia in 2024. And these numbers are still small compared to parts of Africa and Asia with poor healthcare, where measles is much more common (Figure 2).
The problem with measles is that it’s the most contagious airborne virus known to humanity, with an R0 of 12-18. This means that a single infected person can spread the virus to 12-18 others, making even a small drop in vaccination coverage dangerous. While measles typically causes symptoms of fever and rash, it can lead to severe complications like pneumonia, encephalitis, and opportunistic infections, with a fatality rate of 1-2 cases per 1,000 infections. Measles is infamous for erasing immune memory, making people vulnerable to infections they were once protected against.
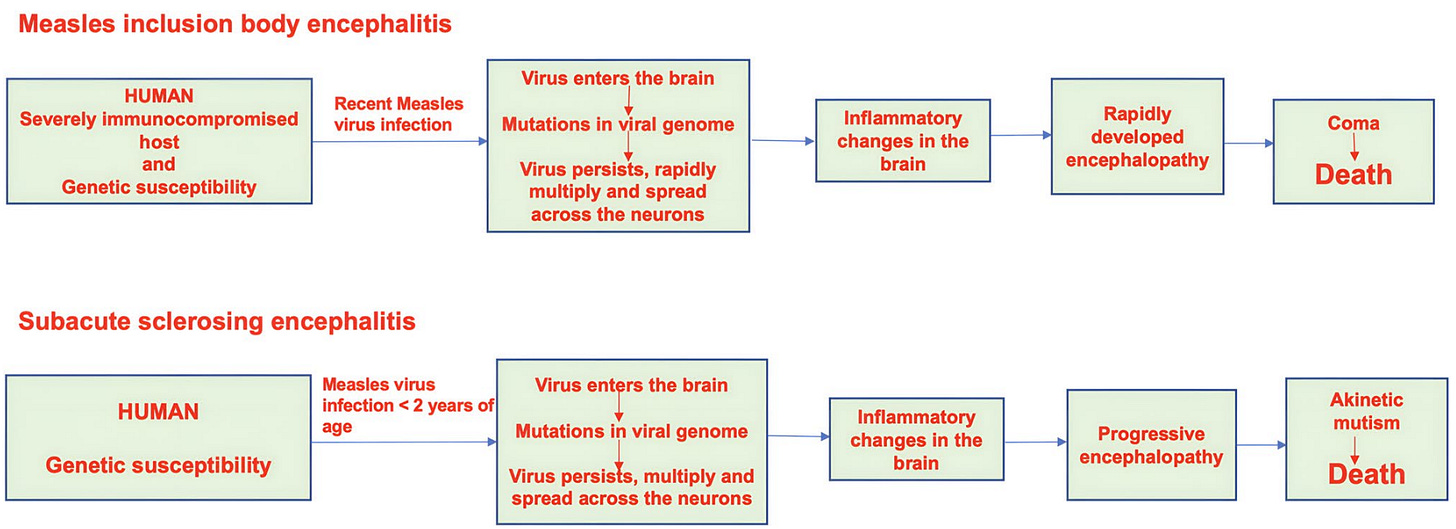
Beyond these commonly known facts about measles, one overlooked aspect is the long-term neurological sequelae of measles, which scream Long Covid all over again. I’ve published academically and written about Long Covid many times, and what I read about ‘Long Measles’ is as, if not more, concerning. It includes severe conditions like subacute sclerosing panencephalitis (SSPE), measles inclusion body encephalitis (MIBE), and acute disseminated encephalomyelitis (ADEM) (Figure 3).
Let’s unpack them one by one.

1. Subacute Sclerosing Panencephalitis (SSPE)
The incidence of SSPE is rare but not negligible. A study conducted during the measles outbreak in the U.S. from 1988 to 1991 estimated that the overall SSPE risk is roughly 1 in 4,635 measles cases. But another review of measles cases in California during the same period reported that the incidence of SSPE is much higher in unvaccinated children under 5 years at the time of measles infection: about 1 in 1,367 cases; similar estimates were reported from Germany at 1 in 1,700 to 3,300 cases.
SSPE develops in four stages, each more severe than the last:
Stage 1: Subtle changes in personality or behavior, such as mood swings, forgetfulness, reduced attention span, or difficulty concentrating.
Stage 2: Neurological symptoms emerge, including frequent muscle jerks (myoclonic jerks), seizures, worsening memory loss, difficulty speaking, vision problems, and weakness in the arms or legs.
Stage 3: Movements become increasingly abnormal (e.g., stiffness, tremors, or involuntary motions), and the person gradually becomes less responsive to people and their environment.
Stage 4: The condition progresses to a vegetative state with complete unresponsiveness. Many patients die from issues such as aspiration pneumonia, sepsis, or respiratory failure, as the brain can no longer sustain basic life functions without medical support.
SSPE is diagnosed by detecting measles-specific IgG antibodies in the cerebrospinal fluid, as well as by abnormal electroencephalograms (EEGs) and brain MRI. A brain biopsy may also reveal measles virus particles inside the nuclei of brain cells. Indeed, the mechanism model of SSPE is the persistence of a mutated measles virus in the brain, which evades immune clearance and progressively destroys neurons from within.
However, this model, proposed as early as 1977, was mainly theoretical (Figure 4). Early work found evidence of the measles virus spreading in the brain, but did not observe any viral budding at the neuronal surface with electron microscopy. So, scientists knew the measles virus could spread in the brain, but they didn't know how or what the mechanism was.

It wasn’t until 2023 that Japanese scientists discovered the neuroinvasion mechanisms of the measles virus. In a series of experiments, Shirogane et al. from Kyushu University in Japan demonstrated that mutations can occur in the F protein of the measles virus. The F protein helps the virus attach to and fuse with cells. Normally, the measles virus can’t infect brain cells, but some variants with a mutated F protein could gain that ability.
What’s extraordinary is that these mutant viruses can drag normal (non-mutated) viruses into the brain along with them via a process called en bloc transmission, where multiple copies of the virus (both mutated and normal) enter a neuron together. Once inside, the different measles virus variants cooperate, enhancing each other’s ability to infect and spread between brain cells. This unexpected teamwork, a phenomenon scientists call “sociovirology,” allows the virus to slowly invade the brain over time, ultimately leading to the devastating symptoms of SSPE.
Put simply, in a rare subset of kids exposed to the measles virus, the virus can mutate and evolve within the body over time into a neuroinvasive form, which can facilitate the en bloc transmission (“en bloc” is French for “as a whole” or “in a block”) of both the mutated and normal measles virus variants between neurons (Figure 5). Over the years, this stealth mechanism can trigger SSPE, causing a slow, progressive, and fatal neurodegeneration.

Once diagnosed, SSPE is almost always fatal. There is no cure, and about 95% of patients die within an average of 3.8 years, with reported survival ranging from just 45 days to as long as 12 years. In the remaining 5%, spontaneous, unexplained improvement has been observed, though long-term remission is exceedingly rare. The reasons behind this remain unclear but likely involve a complex interplay of viral, immune, and genetic factors.
Before moving on to the next neurological sequelae, here’s a short documentary about Sarah Walton, a healthy baby who contracted measles at 11 months old. At the time, she recovered quickly and went on to lead a fulfilling life. But in 2004, 25-year-old Sarah began facing unexplained health issues. She was later diagnosed with SSPE and became bedbound due to severe neurological deterioration. Her mother, Jo Walton, later reflected on the decision not to vaccinate Sarah against measles due to concerns over egg allergies. In hindsight, Jo deeply regrets not being informed about SSPE, a condition she describes as life-shattering:
2. Measles Inclusion Body Encephalitis (MIBE)
Another severe post-measles neurological sequelae is measles inclusion body encephalitis (MIBE), a rapidly progressive and often fatal brain disease that occurs in immunocompromised individuals, particularly children with conditions such as lymphoblastic leukemia (a type of blood cancer), HIV/AIDS, autoimmune disorders, or genetic immunodeficiencies, as well as those undergoing solid-organ or stem cell transplantation.
Unlike SSPE, which develops about a decade after infection, MIBE usually develops within a year of measles infection. Clinically, MIBE often begins with seizures that become increasingly frequent and difficult to control, eventually leading to progressive brain dysfunction (encephalopathy), coma, and death. Other symptoms may include involuntary jerks, vision loss, dysphagia (difficulty swallowing), and loss of muscle control.
Neuroimaging typically reveals hyperintensity abnormalities in both white and gray matter, although some patients may have normal scans early on. Brain biopsy or autopsy remains the gold standard for diagnosis, revealing measles virus proteins within neuronal and other brain cells (Figure 6). Similar to the SSPE mechanism model, mutations in the F (fusion) protein of the measles virus are believed to drive neurovirulence of MIBE by facilitating neuron-to-neuron spread and immune evasion (Figure 7).

Despite sharing pathological features with SSPE (e.g., presence of measles virus as intraneuronal inclusions in the brain), MIBE is clinically distinct. For instance, MIBE begins with seizures that worsen over time, whereas SSPE begins with personality and behavioral changes before the cognitive and motor deficits develop. SSPE usually affects immunocompetent children and progresses slowly over a decade, while MIBE occurs in those who are immunocompromised and quickly leads to death within a year.
MIBE also carries a terrible prognosis, with a mortality rate exceeding 90%. As no effective treatment exists, supportive care is the only option. Similar to SSPE, survivors of MIBE are often left with neurological disabilities in the long run. However, given the scarcity of MIBE cases and survivors, only case reports have documented its long-term outcomes.
In fact, in a 2024 systematic review of 39 case reports/series on 49 MIBE patients, only 4 patients managed to survive with long-term neurological disability, putting the survival rate at just 8%. These 4 patients are:
Turner et al. (2006), UK: An 8-year-old boy with MIBE showed improvement over 12 months after intense treatment. Although he regained mobility, he continued to face problems with balancing, speech, and swallowing due to residual neurological damage.
Turner et al. (2006), UK: The second patient, a 13-year-old boy, had a much poorer outcome. Two years after MIBE, he remained wheelchair-bound, registered blind, and had limited ability to communicate, reflecting the permanent neurological impact of MIBE.
Mustafa et al. (1993), US: Despite surviving MIBE, a 4-year-old girl in Texas was left with severe long-term neurocognitive impairments. She regained physical functions like walking, eating, and hearing, but remained unable to speak and cognitively inept two years later.
Kurtzke (1956), US: A 20-year-old man in New York survived MIBE after months of coma and severe neurological impairment. Two years later, he regained some speech, mobility, and partial independence in eating and sensory functions, but remained amnesic (memory loss), disoriented, emotionally unstable, and faced recurrent seizures.
“He still is amnesic, anosognosic, disoriented to time and place, and emotionally labile. Recent memory is poor. He gives his age, at 22, as 16. He repeats three numbers forward and reversed. His production on serial sevens is: “100 - 96 - 89 - why? why?”. He can count to ten and add 2 lus 2. General information is markedly limiteb: Grand ma1 seizures occur at irregular intervals despite anticonvulsant medication, averaging one series of several consecutive seizures every four months.” — Kurtzke (1956).
3. Acute Disseminated Encephalomyelitis (ADEM)
Another serious neurological complication linked to measles is acute disseminated encephalomyelitis (ADEM), an inflammatory condition in which the immune system mistakenly attacks the brain and spinal cord.
ADEM usually develops within days to weeks after infection and causes widespread demyelination, where the myelin sheath that insulates nerve fibers is stripped away. This causes motor and sensory deficits, such as vision loss, muscle weakness, loss of bowel or bladder control, tingling, seizures, and even coma (Figure 8). MRI scans typically show scattered white matter lesions throughout the central nervous system. Elevated levels of myelin basic protein, indicative of myelin sheath damage, in the cerebrospinal fluid further support the diagnosis.

Measles is a well-known trigger of ADEM. In fact, ADEM accounts for a significant proportion of the acute encephalitis seen during or shortly after measles infection, occurring in about 1 in 1,000 cases. The mechanism is not due to direct viral invasion, but rather to an autoimmune misfire, where the immune system confuses viral antigens with components of the nervous system, particularly myelin, and launches an attack.
While ADEM can also occur following Covid-19, it is much rarer compared to post-measles. These parallels highlight an important point: post-viral autoimmune attacks on the brain are not unique to Covid-19. Measles has been doing this for decades, and with greater frequency.
Treatment for ADEM focuses on modulating the immune system rather than clearing a virus. While the clinical outlook for ADEM is generally more favorable than for SSPE, ADEM still carries a mortality rate of 10-20%. The average recovery time ranges from 1 to 6 months, but some patients may be left with long-term neurological impairments.
For instance, a 2019 nationwide Danish study of 52 children with ADEM diagnosed in 2008–2015 found that about 40% still had residual white matter abnormalities on brain MRI at a median follow-up of 8 years. These lingering lesions suggest that the brain does not always fully heal, even without obvious disability. While the study reported that academic outcomes were unaffected, ADEM survivors had a 2.4-fold increase in sleep disturbances and depression compared to controls.
Other studies have reported more severe long-term outcomes in ADEM survivors. For example, a 2022 Argentinian cohort of 84 children with ADEM, followed for an average of 6.6 years, found that about 11% were left with moderate to severe disability, including hemiparesis (weakness of one side of the body), visual loss, epilepsy, or cognitive delays. These outcomes were not predicted by initial brain MRI findings, suggesting that lesion size at onset may not fully capture the risk of long-term impairment.
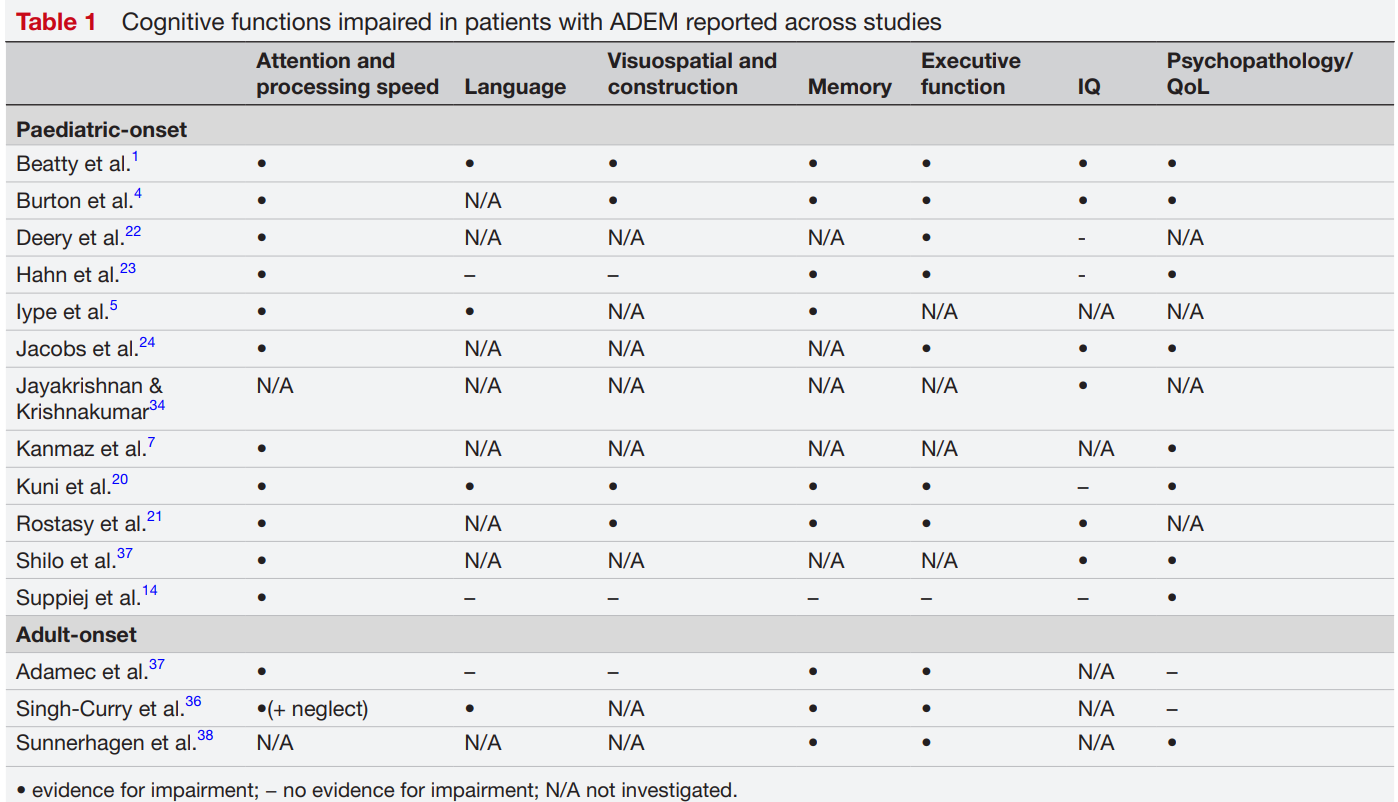
A 2017 meta-analysis of 13 studies concluded that long-term cognitive impairment was uncommon among ADEM survivors at the group level. There was a statistical trend towards reduced processing speed and increased emotional disorders though (p = 0.06). But at the individual level, 20–40% of patients showed subtle but persistent deficits in attention, executive function, memory, or emotional regulation. In other words, while most patients with ADEM recover without overall cognitive decline, some are left with long-term issues in at least one cognitive domain.
“There is emerging evidence to suggest that individuals—particularly children—with ADEM experience persistent cognitive deficits and suffer from elevated symptoms of depression and anxiety,” argued Kazzi et al. in a 2024 literature review that critically examined the available body of research on the long-term cognitive outcomes of ADEM survivors (Figure 9).
Parallels to Long Covid
The long-term neurological consequences of measles infections are eerily reminiscent of what we now know as Long Covid. Both involve a post-viral phase where an initial infection leads to a cascade of biological abnormalities that persist for months and even years. In Long Covid, the range of abnormalities is broader, encompassing both neurological and non-neurological problems. In “Long Measles,” only neurological functions are affected, but they are often more severe and cause disabilities.
Regardless of their differences, Long Covid and Long Measles share a key characteristic: lasting damage to the brain. As brain neurons don’t readily regenerate, any damage is often persistent. In Long Covid, persistent brain imaging abnormalities have also been detected, even raising biomarkers of neuronal damage and inflammation in the cerebrospinal fluid.
While the term “Long Measles” hasn’t entered mainstream medical discourse the way Long COVID has, it arguably deserves to. Like SARS-CoV-2, the measles virus doesn’t always leave quietly after the acute illness ends. Instead, it can initiate a cascade of long-term effects that linger for months or even years. Survivors—especially children—may experience persistent cognitive impairment, behavioral changes, and increased vulnerability to infections due to the virus’s ability to erase immune memory.
In some, measles triggers autoimmune attacks on the brain, as seen in acute disseminated encephalomyelitis (ADEM). In others, the virus remains dormant for years before reactivating as subacute sclerosing panencephalitis (SSPE). And in severely immunocompromised individuals, the virus may never be fully cleared at all, leading to a rapid and often fatal brain infection known as measles inclusion body encephalitis (MIBE).
Taken together, these outcomes resemble a post-viral syndrome in everything but name. So while “Long Measles” may not be a formal diagnosis, the concept captures something very real: the measles virus has a long tail, and for some, that tail can be devastating if not fatal (Figure 3).
For re-emphasis, let me put Figure 3 here again:

This article was initially published in Microbial Instincts on Medium’s annual draft day on April 25, 2025, so I’ve made it free to read here on Substack.
If you've made it this far, thank you for reading! Most of my Substack articles are paywalled, so if you found this valuable, consider subscribing for just $2.90/month (annual plan). Your support allows me to dedicate more time to researching and writing about lesser-known but important topics like this.





Thank you for posting this on Substack and without a paywall. I share your concerns. It’s very informative and I hope you don’t mind my sharing it. Thank you, John.
Hidden within the familiar ache of illness lies a quieter story — of immune memory erased, of bodies struggling to remember the songs of protection they once knew. Like rivers after a drought, the immune systems touched by measles and its long echo seem to flow with less certainty, searching for their former strength. This reminds us how delicate our woven shields are, and how compassion for those facing unseen battles becomes an essential medicine of its own.
In the fragile dance between past infections and future resilience, we are called to tend not just our bodies, but the communal garden of health we share. How might we, together, strengthen the unseen roots that nourish our collective well-being? ∞