Most Long COVID Biomarkers Are Not That Useful, But One Truly Stands Out
My observations as an academic who has published in this area.
This article was originally published at Microbial Instincts on January 28, 2024.
Each time a major study announces the discovery of new biomarkers for long COVID, the news rapidly ignites across media platforms, sparking widespread excitement that we may finally be able to test whether someone truly has long COVID or not.
But are things that simple? Will science finally reach a point where a simple blood test can determine a long COVID diagnosis? I find these questions tricky to answer, which is why I’m writing about them.
Recently, several important long COVID biomarker studies have been published in top academic journals like Nature, Science, and Cell. Even I’ve also published in this area, specifically a meta-analysis of over 20 potential biomarkers of long COVID. I’ll delve into these studies with a critical eye to understand what they mean in the real, clinical context.
Author’s note: Feel free to skip to the last section for the main takeaways.
Biomarker 1: Complement proteins
The latest long COVID biomarker study, titled “Persistent complement dysregulation with signs of thromboinflammation in active Long Covid,” was published in Science earlier this year. It’s conducted by Cervia-Hasler et al. from top institutions in America, Switzerland, and Sweden.
This study tracked 39 healthy individuals and 133 Covid-19 survivors, of whom 40 survivors developed long COVID at 6-month. During each clinical evaluation, a blood sample was drawn, resulting in a total of 268 longitudinal blood samples for analysis. Over 6,500 proteins in these blood samples were analyzed using machine learning methods.
Results showed those who developed long COVID had increased activation of the complement system during Covid-19 — with increased levels of C5bC, Factor Ba and C2, but decreased levels of C7 complement proteins— which remains activated at 6-month. But, the complement proteins normalized in Covid-19 survivors who did not develop long COVID (Figure 1 and 2).
However, the problem with these data is the huge overlaps in the levels of C7 and C4bC between Covid-19 survivors who did not develop long COVID, who recovered from long COVID, and who had ongoing long COVID.


This means that the clinical effect size is minimal, even though it reaches statistical significance (marked by ** or *** in the figure). So, if a long COVID patient gets his/her C7 levels measured and it doesn't show a significant decrease, it still cannot rule out a long COVID diagnosis.
I’ll not delve into the complicated complement system in detail here. In general, it’s a part of the immune system that enhances (complements) the ability of other immune cells to clear foreign threats and cell debris.
Biomarker 2: Thromboinflammation
The same study in Science by Cervia-Hasler also found increased biomarkers indicative of tissue injury and thromboinflammation. Specifically, compared to no long COVID, those with long COVID had:
↓ blood levels of hemopexin, antithrombin III, ICAM-1, S100-A8/A9, ADAMTS13, and platelet-activating factor acetylhydrolase (PAF-AH).
↑ blood levels of von Willebrand factor (vWF), hepatoglobin, factor VII, thrombospondin-1 (TSP-1), interleukin-6 (IL-6), and lactate dehydrogenase (LDH).
I’ll not discuss what each of these biomarkers represents. In brief, they are simply various components of the thrombo-inflammation cascade, i.e., inflammation of blood vessels, which can cause tissue injury. The complement system also influences this process.
But what I want to point out is, again, levels of these biomarkers related to thromboinflammation biomarkers greatly overlap between those with and without long COVID. A few examples are depicted in Figure 3:

In my published meta-analysis of 23 studies, titled “Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers,” I also found increased levels of several biomarkers involved in thromboinflammation, such as C-reactive protein (CRP), D-dimer, IL-6, and LDH.
However, the calculated effect sizes in my meta-analysis were all small, with standardized mean difference (SMD) values of 0.2–0.3, with >0.5 considered moderate and >0.7 considered large effect sizes. Examples of CRP and D-dimer are depicted in Figure 4. This means that levels of such biomarkers, albeit statistically significant at the meta-analytical level, still greatly overlap between those with and without long COVID.

Biomarker 3: Cortisol
Another major long COVID biomarker study is by Klein et al. from Yale University, published in September 2023 in Nature, titled “Distinguishing features of long COVID identified through immune profiling.”
This study recruited 275 individuals with or without long COVID in a simple cross-sectional design (i.e., capturing data at one time point, unlike the longitudinal design by Cervia-Hasler et al.) and subjected their blood samples to machine learning analyses to identify potential biomarkers.
First, Klein et al. found several differences in the immune system of long COVID participants, characterized by altered levels of certain immune cells (i.e., monocytes, dendritic cells, B-cells, and T-cells) and increased levels of antibodies against SARS-CoV-2 and other herpesviruses.
However, the biomarker that stood out the most was cortisol, a hormone involved in stress response, which their machine learning analyses deemed the strongest predictor of long COVID (Figure 5A). But even so, the cortisol levels still highly overlapped between those with and without long COVID, even though the difference was statistically significant (Figure 5B).
Klein et al.’s study also found increased levels of complement protein C4b and other inflammatory mediators like chemokines CCL19, CCL20, and CCL3. But again, their data points, though statistically significant, highly overlap between those with and without long COVID (Figure 6).


Biomarker 4: Serotonin
Now, let’s move on to the real deal. Serotonin, a common neurotransmitter, is arguably by far the best biomarker of long COVID. Let me explain.
Published in Cell in October 2023, Wong et al. from the University of Pennsylvania and the University of California performed a breakthrough study titled “Serotonin reduction in post-acute sequelae of viral infection.”
First, they analyzed publicly available metabolomic data from five published studies on Covid-19 patients vs. healthy controls, showing that amino acids were the most altered metabolites in Covid-19 patients. They then analyzed these metabolites in a new cohort of 1,540 individuals with long COVID, who were grouped into 8 subtypes based on symptom clusters (e.g., mobility, fatigue, cardiorespiratory, neurocognitive, etc.).
Next, they selected 58 long COVID patients representative of the various subtypes for further metabolomic analyses of their blood samples. These patients had long COVID symptoms for 3–22 months. They also included 60 patients with ongoing Covid-19 and 30 Covid-19 survivors without long COVID as the control groups from Pennsylvania, U.S.
Metabolomics is the study of metabolites — the small molecules involved in cell metabolism processes — present in a certain biological sample.
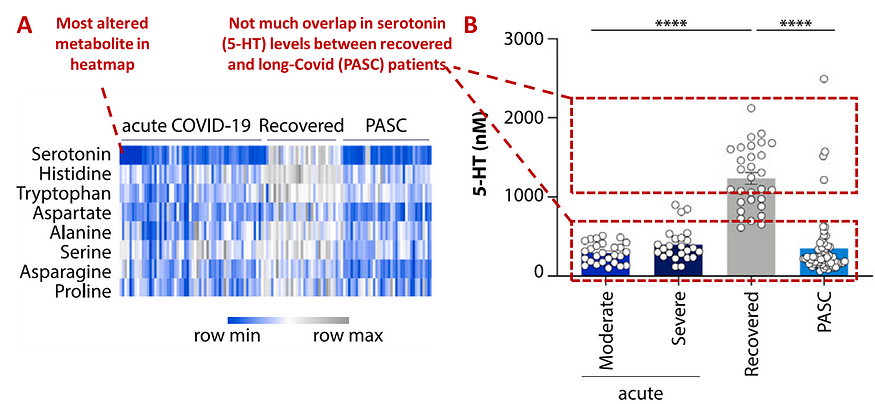
Results showed that serotonin was the most significant depleted metabolite in patients with ongoing Covid-19 and with long COVID. Following acute Covid-19, serotonin was also the greatest predictor of whether a patient fully recovered or developed long COVID (Figure 7). Other amino acids were either unaltered in patients or normalized following acute Covid-19. Wong et al. also replicated the serotonin reduction association with long COVID in two separate cohorts from Cork, Ireland and California, U.S.
As the biomarker plot shows, the individual serotonin levels did not really overlap between Covid-19 survivors with and without long COVID. Serotonin levels were also similar between patients with moderate/severe ongoing Covid-19 and with long COVID — indicating that serotonin reduction occurred during acute Covid-19 and remained reduced in long COVID.
What’s more remarkable is that Wong et al. further did a series of lab experiments to delineate the causative process of how serotonin reduction might cause long COVID. I’ll not discuss them in detail here as they are worth a separate article, but here’s the gist of their findings:
Serotonin levels were also decreased in individuals with non-Covid infections— such as adenovirus, rhinovirus and respiratory syncytial virus (RSV) — compared to healthy individuals, suggesting that serotonin reduction is a general complication of viral infections.
Infecting mice with acute (SARS-CoV-2 or VSV, vesicular stomatitis virus) or persistent (LCMS, lymphocytic choriomeningitis virus) virus infections also led to serotonin reduction in the blood.
Imitating viral inflammation with synthetic poly(I:C) — which mimics viral replication intermediates — also reduced serotonin levels in mice. Serotonin levels were restored within a week of poly(I:C) cessation.
Indeed, exposure to SARS-CoV-2 or VSV infection, LCMV persistent infection, or poly(I:C) injection all increased the activities of pro-inflammatory interferons — indicating that serotonin reduction is a result of unresolved viral inflammation.
Low tryptophan levels were also noted in humans and mice exposed to virus infection or poly(I:C) injection. Tryptophan, an amino acid found in food, is needed for serotonin synthesis. More experiments found that persistent virus infection and inflammation in the gut hinder the absorption of dietary tryptophan, reducing serotonin synthesis.
Further experiments showed that viral inflammation drives vascular dysfunction and decreased white blood cells, including platelets. Platelets are needed for blood clotting and serotonin storage, so their deficiency also contributes to serotonin reduction.
They also found that serotonin reduction impairs the vagus nerve signalling connecting the gut and brain in mice. But treatment with antidepressants (selective serotonin reuptake inhibitor, SSRI) or tryptophan supplements restored cognitive function in the mice.
Wong et al. also nicely summarized the mechanism of how virus infection can trigger a series of biochemical pathways leading to impaired neuro-cognitive functions here (Figure 8):

What does it all mean
Among the hallmark long COVID biomarker studies published in top journals like Science, Nature, and Cell, only the Cell study is actually helpful in showing how serotonin levels in the blood plasma can differentiate between individuals with and without long COVID.
The other biomarkers — namely complement proteins, thrombo-inflammation mediators, and cortisol — overlap between individuals with and without long COVID to a great extent. This makes their effect sizes small and less helpful in identifying actual long COVID patients.
But studies are often reluctant to admit if their effect sizes are small, for it undermines their labor and research’s value. The funders and public won’t be so happy to hear about small effect sizes. The general media or news outlet won’t be able to pick such a nuanced detail either, making us all believe that the holy grail of long COVID diagnostics is actually here.
Peter Miller, a fellow science writer, pointed out the same problem in his older work, “The CellTrend test won’t help you diagnose long covid.” Herein,he highlighted the flaw with a common long COVID test on the market, created by CellTrend, a German company. CellTrend measures ACE2-targeting antibodies based on the theory of ACE2 dysregulation in long COVID.
But Peter Miller cited studies showing that even healthy individuals can have ACE2 antibodies out of the normal range as indicated by CellTrend. So, a healthy person may actually get diagnosed with long COVID if the CellTrend test is given medical validity. Peter Miller also pointed out more issues with long COVID diagnostic research that are still relevant today, so I really recommend reading his article here:
Despite serotonin's promising potential as a long COVID biomarker, some challenges still remain. For one, reduced serotonin could also indicate the presence of other diseases, such as depression and other neuropsychiatric disorders. Second, serotonin is not included in routine blood tests, making it difficult to standardize serotonin tests across institutions.
Third, the study authors admit that serotonin levels could differ among long COVID subtypes. While their research incorporated a representative sample from each identified long COVID subtype, the highly diverse nature of the condition means that not every subtype may have been included in their study. In my own meta-analysis paper, I highlighted that no universal biomarker may exist for this multifaceted condition.
That said, my perspective could be wrong. As further research unfolds, serotonin might indeed become a viable tool for clinical diagnosis of long COVID. The brilliant study by Wong et al. is a great start.





