Amyloids: What Evolution Reveals About Them and Why They Turn Against Us
On the amyloid paradox (part III)
For billions of years, amyloid fibrils served as architects of life. They shielded fragile molecules from the harsh environment of early Earth, provided structural resilience to microbial life, and even shaped protein inheritance mechanisms in certain fungal species.
Yet, in an apparent act of betrayal by evolution, the very properties that once made amyloids indispensable—such as mechanical resilience, self-replication, and stability—have turned against us. In the human body, these structures misfold and accumulate as toxic aggregates to cause diseases. Instead of supporting life, they now dismantle it.
This paradox raises the question: Why did evolution allow amyloids to persist if they cause lethal diseases? If natural selection is meant to weed out harmful traits, why do we still inherit amyloid proteins? This final third part of the amyloid paradox series will address these questions.
(Read part I here and part II here.)
On the Various Kinds of Cytotoxic Amyloids
First, let’s clarify that cytotoxic amyloids mean amyloids that are toxic to cells (cytotoxic). But not all cytotoxic amyloids are self-disruptive.
Some cytotoxic amyloids are self-beneficial, as discussed in parts I and II. For instance, bacteria form amyloid-supported biofilms for their survival, but at the expense of other organisms in cases of bacterial infections. Bacterial biofilms on medical devices or dental plaques help the bacteria survive, but not the infected host. Moreover, some bacterial species produce amyloid-based bacteriocins to kill other competing bacteria. So, despite their cytotoxicity, these amyloids still benefit the self.
In contrast, self-disruptive cytotoxic amyloids serve little to no beneficial functions to the self and are ultimately self-disruptive. The most infamous example of this is the amyloid-β (Aβ) plaques seen in the brain of patients with Alzheimer’s disease (AD), the most common neurodegenerative disease. Other examples of self-disruptive cytotoxic amyloids include:
Prion Proteins: When prion proteins misfold into a β–sheet–rich conformation, they form infectious amyloid fibrils that underlie prion diseases such as Creutzfeldt–Jakob disease (CJD) in humans and scrapie in sheep. It’s infectious because the misfolded prion proteins rapidly initiate the misfolding of other prion proteins.
Alpha-Synuclein: In Parkinson’s disease and related synucleinopathies, α-synuclein proteins aggregate into fibrils that form Lewy bodies. These toxic fibrils degenerate dopaminergic neurons at the substantial nigra, a brain region responsible for controlling body movement.
Tau Aggregates: Alongside Aβ plaques, neurofibrillary tangles consisting of misfolded tau proteins represent the two major hallmarks of AD. Although often considered secondary to Aβ plaque, tau aggregates are very cytotoxic and cause other tauopathies, such as Pick disease, progressive supranuclear palsy, and corticobasal degeneration.
Islet Amyloid Polypeptide (IAPP): The IAPP, also known as amylin, forms amyloid deposits in the pancreatic cells of patients with type 2 diabetes. These cytotoxic deposits impair insulin secretion.
Light Chain Amyloidosis: Misfolded immunoglobulin light chains can aggregate and deposit in various tissues, such as the heart and kidneys. These deposits disrupt normal organ function and can cause systemic amyloidosis, harming multiple organ systems.
So, Aβ plaques are not the only self-disruptive cytotoxic amyloids. Despite prion proteins, α-synuclein, tau, and others not having ‘amyloid’ in their names, they can also misfold and aggregate into fibrils rich in β-sheet structures (Figure 1). As defined in part I, mechanically resilient β-sheet structures are the defining feature of amyloid fibrils.
That said, among these self-disruptive cytotoxic amyloids, Aβ plaques remain the most studied due to their causative nature in AD, one of the most devastating diseases known to humanity. Much of this article will use Aβ plaques as the central example to decode the amyloid paradox.

The Evolutionary Origin and Function of Aβ Plaques
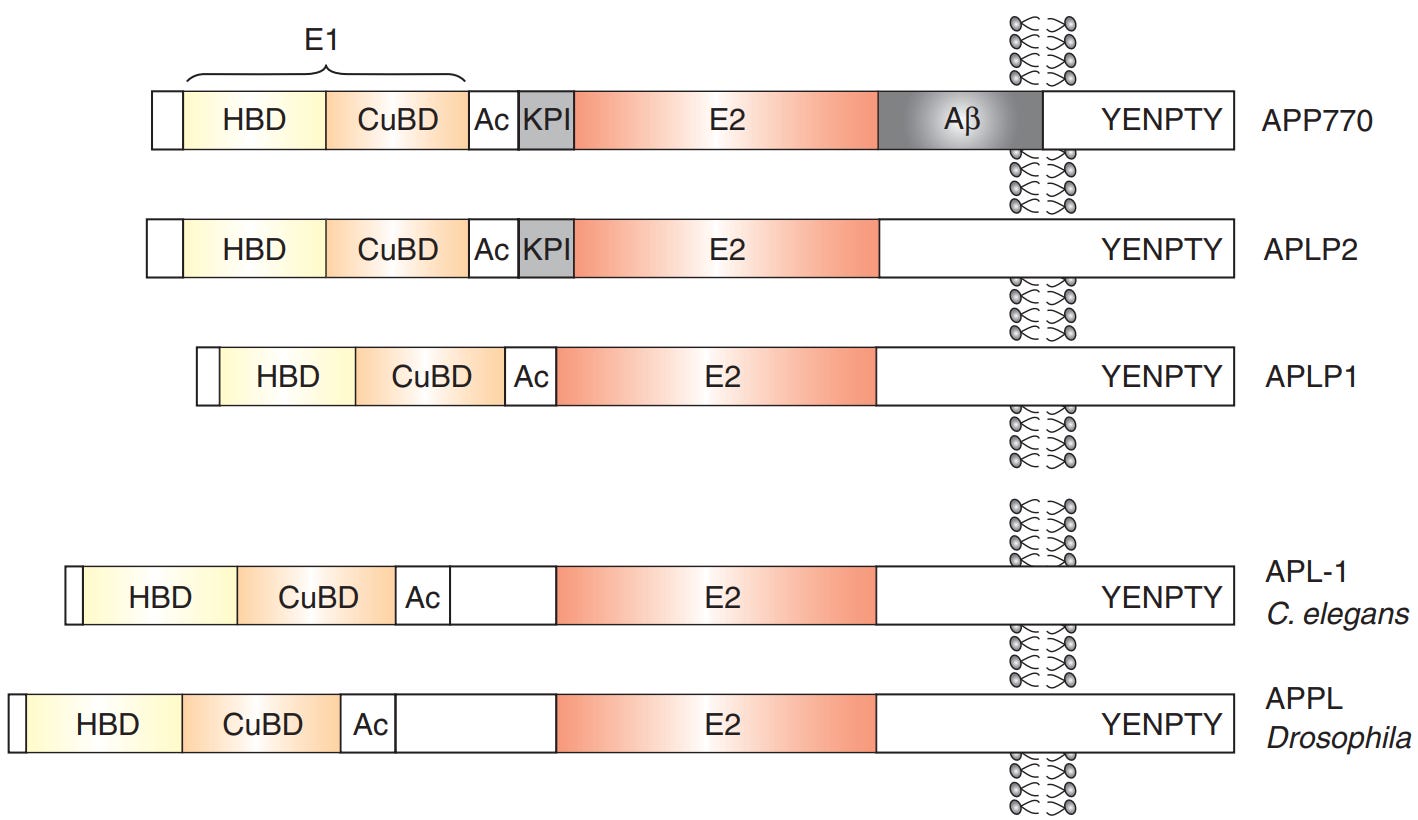
For context, Aβ plaques are aggregated Aβ peptides, a by-product of the abnormal processing of the amyloid precursor protein (APP) gene.
The APP gene is ancient. Phylogenetic analyses by Tharp and Sarkar (2013) from the University of Vermont, US, reveal that APP-like genes date back at least 540–630 million years when the first complex animals—marine invertebrates like sea anemones and hydra—first evolved (Figure 2).
But these APP-like genes don’t produce cytotoxic Aβ in any invertebrates (animals without backbones), unless they are artificially overexpressed or mutated in the laboratory to create animal models of AD.

These ancient APP-like genes must, or at least very likely, serve some purpose. Otherwise, why would evolution keep them to this day?
To this end, Coulson et al. (2000) from the University of Melbourne found that APP originally functioned as a cell adhesion molecule by studying how the gene family evolved across species. By looking at which part of the APP gene stayed the same over millions of years, they found that certain regions, especially those linked to cell attachment, were highly conserved. This suggests that these parts must be important for the protein’s original role, which would likely help cells stick together and grow properly.
To support this, Coulson et al. cited previous experiments in which APP was suppressed or removed from cells. These studies showed that without APP, neurons had trouble growing and forming connections (synapses). After all, neurite outgrowth relies on adhesion to the extracellular environment and neighboring neurons to form synapses (Figure 3).

So, the APP gene family is initially self-beneficial, helping neurons form synapses. But at some point in evolution, the APP gene began producing self-disruptive cytotoxic amyloids, i.e., Aβ plaques.
To pinpoint which point, we can look at the evolution of the βA4 sequence, the part of the APP gene that produces Aβ peptides (Figure 4). Coulson et al. also found that this βA4 sequence is relatively recent but is still highly conserved across species, from puffer fish to humans.
The βA4 sequence is still important for the APP gene to function properly, though. Experiments showed that removing the βA4 sequence from the APP gene caused the APP to take the wrong path instead of being transported to the neurite axons. This suggests that the βA4 sequence acts like a built-in taxi driver, transporting APP towards the neurite axons so that APP can mediate cell adhesion and synaptic formation.
Amyloids are initially characterized by their exceptionally resilient β-sheet structures. Although the exact mechanism by which these structures evolved to mediate neuronal synaptic formation remains unclear, we can speculate that their inherent stability was repurposed over time to serve as a reliable structural framework in more complex organisms.
In the context of cell adhesion, this durability may be crucial. For example, when APP is transported to neurite axons, its amyloid-like domains may form a robust scaffold that supports the formation of synaptic connections, even under the dynamic forces of the extracellular (outside of cells) environment.
The Evolutionary Turning Point of Aβ plaques
The advent of the APP gene’s βA4 sequence also matches the evolutionary turning point at which organisms began developing Aβ plaques naturally.
We can exclude invertebrates, reptiles, and amphibians that don’t form Aβ plaques naturally. The only indications of natural Aβ plaque formation have been observed in one species of fish (salmon), some species of birds (e.g., woodpeckers and grey parrots), and numerous species of mammals (e.g., guinea pigs, rabbits, dogs, cats, cheetahs, whales, dolphins, seals, cattle, wolverine, and non-human primates like chimpanzees).
Among these, salmon is the oldest, having evolved about 50-100 million years ago, which is also consistent with the emergence of the βA4 sequence in puffer fish 80-100 million years ago. So, the evolutionary origin of Aβ peptides and plaques—generated from the βA4 sequence of the APP gene—can be traced back to fish, particularly salmon.
Indeed, one of the earliest evolutionary indications of self-disruptive Aβ plaque is in salmon. This species exhibits semelparity in its programmed life cycle, where it spawns (reproduces) once and then dies (Figure 5). While this trait benefits the species by preventing competition for food with offspring, it’s evidently self-disruptive. Although hormonal changes are the primary trigger of semelparity in salmon, widespread Aβ plaques have also been found in the degenerating brain tissues of salmon during and after spawning.

What went wrong with salmon, then? Nothing, actually. Scientists argue that amyloids are still considered ‘functional’ in salmon because they are programmed in their life cycle. So, every salmon will develop Aβ plaques. In this manner, the cytotoxic amyloids in salmon are not pathological, as they don’t cause diseases in some members of the species. Put simply, if everyone has it, it’s more reasonable to say that nothing ‘went’ wrong.
The better question to ask is: What went wrong in the species where self-disruptive Aβ plaques are pathological (disease-causing)?
We can begin by eliminating birds. Only two species of birds have been found to show natural Aβ plaque formation in their brains, but this is not accompanied by overt neurodegeneration or cognitive deterioration. (Notably, numerous bird species do suffer from systemic or multi-organ amyloidosis, but this disease has a different pathology than AD.)
This leaves us with mammals as the evolutionary turning point at which amyloids became pathologically self-destructive. Why?
Keep reading with a 7-day free trial
Subscribe to The Infected Neuron to keep reading this post and get 7 days of free access to the full post archives.





