Our Neurons Mastered Electrical Signals 450 Million Years Ago — Thanks to a Virus Infection
On the marvelous origin of the neuron’s myelin sheath.
This article was originally published at Microbial Instincts on August 16, 2024.
Around 430–450 million years ago, sea creatures began evolving jaws, bony structures that assist biting. It was also during this time that myelin sheaths first appeared in jawed creatures.
What exactly connected the co-evolution of jaws and myelin sheath is unclear, though there are possible theories I’ll explore later.
Importantly, have you heard about the myelin sheath, the magnificent structure unique to the nervous system? Without it, our nervous system wouldn’t be what it is today—compact and efficient.
Look at some complex species without myelin sheaths, like squids and octopuses. Their unmyelinated nerve fibers take up 15,000 times more space than a similarly conducting myelinated nerve in mammals and consume thousands of times more energy.
If we apply this to humans, the human spinal cord would need to be almost a meter wide if the myelin sheath didn’t exist. Can you imagine our lower back’s width (not diameter) being more than 100 cm (Fig 1)?

Now, how did the evolution of myelin sheath occur?
Believe it or not, it started with a virus infection.
The Origin of the Myelin Sheath
Before proceeding, let’s ensure we know what the myelin sheath is.
Basically, it’s like the insulation around electrical wires. Because the myelin sheath doesn’t conduct electricity, it forces electrical signals to “jump” from one gap in the insulation to the next along the nerve fiber. These gaps are called nodes of Ranvier, the areas in between myelin sheaths where the nerve fibers are unmyelinated (Fig 2). This jumping mechanism allows electrical signals to travel rapidly and efficiently.
In unmyelinated fibers, signals move at a slower pace of 0.5 to 10 meters per second, while myelinated fibers can reach speeds of up to 150 meters per second — dramatically enhancing the speed of neural signals.

Scientists have long wondered how the myelin sheath evolved. Unlike other structures that evolve gradually, the myelin sheath suddenly appeared in jawed vertebrates around 430–450 million years ago without any preexisting structures that could give rise to it.
It wasn’t until recently that concrete evidence of the myelin sheath’s evolution was uncovered in a 2024 paper by Ghosh et al. from the University of Cambridge, UK, published in the journal Cell.
How they tracked the evolution of the myelin sheath was simple yet ingenious. They first tracked which gene was highly expressed during the formation of the myelin sheath, followed by experiments showing that the gene is essential for myelin sheath formation, and, finally, genetic analyses to track the evolutionary origin of the gene.
The gene in question is RNLTR12-int/RetroMyelin.
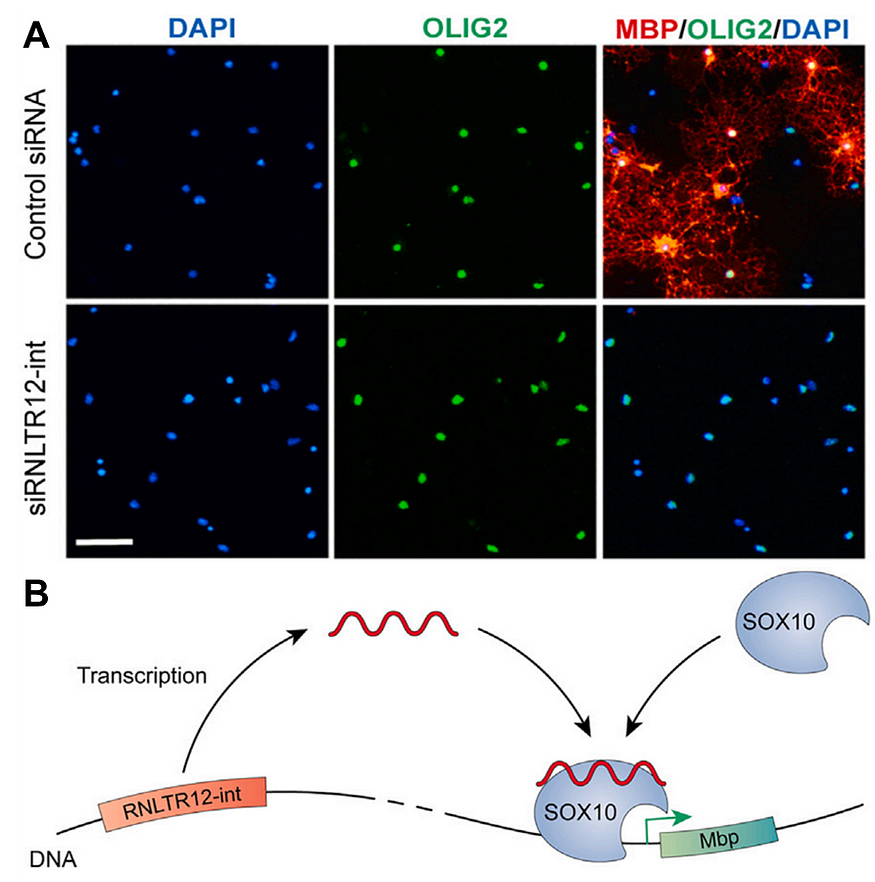
Ghosh et al. found that this gene was highly expressed alongside myelination-related genes in the developing brains of rats. When Ghosh et al. silenced this gene in baby rats, the expression of myelin basic protein (MBP) and other myelination-related genes was inhibited, and the myelin sheath didn’t form (Fig 3A). Similar findings were observed with zebrafish and frogs. Further experiments showed that this gene binds to another gene called SOX10, a necessary step to activate MBP (Fig 3B).
Overall, these findings establish the indispensable role of the RNLTR12-int gene in the formation of myelin sheath across various species. With this, Ghosh et al. redefined RNLTR12-int as RetroMyelin (retrotransposon sequences associated with myelin evolution).
And now things get more fascinating.
RNLTR12-int/RetroMyelin is actually a gene from the family of endogenous retrovirus 1 (ERV1). These are ancient retroviral sequences that have become a permanent part of the host organism’s genome.
Retroviruses are a class of viruses that includes human immunodeficiency virus (HIV). They can reverse-transcribe their RNA into DNA to be integrated into the DNA of the host genome. If a retrovirus infects the reproductive cells (sperm or egg), its genes are permanently integrated into the host genome and passed down from generation to generation.
This means the evolution of myelin sheath was possible because a retrovirus infected our ancestors hundreds of million years ago.
As Ghosh et al. wrote, “In the present study, we propose that in vertebrate species, RetroMyelin sequences originate due to germline invasion of ancient retroviruses carrying an RNLTR12-int-like sequence.”
“In summary, our study suggests that RetroMyelin was co-opted to regulate transcription of [myelin basic protein]; thus, endogenization of ERV1 into the vertebrate genome is coupled to the evolutionary emergence of myelination, a critical step in vertebrate evolution.”
Evolution of the Myelin Sheath
Ghosh et al. then investigated the presence of RNLTR12-int/RetroMyelin in living creatures, including extinct and ancestral species. Per prior research, this gene is present in all jawed vertebrates, including bony fish (Fig 4), amphibians, reptiles, birds, and mammals.
But the gene is absent in jawless vertebrates (lamprey, Fig 4) and invertebrates (fruit flies, worms, and sea anemones). Vertebrates have backbones, while invertebrates do not.

This means either one of two things:
Single event before speciation: All vertebrates inherited RNLTR12-int/RetroMyelin from a common ancestor of jawed vertebrates before they evolved into different species.
Multiple events after speciation: RNLTR12-int/RetroMyelin entered the DNA of each species separately, i.e., after they had become distinct species.
Things get more complicated here.
If the single-event scenario were true, the RNLTR12-int/RetroMyelin sequences would start to differ as species evolved, and they wouldn’t group together neatly when we look at their genetic family tree.
If the multiple-event scenario were true, the RNLTR12-int/RetroMyelin sequences within each species would be more similar and cluster together more neatly on the genetic tree.
To determine which scenario was true, Ghosh et al. grouped RNLTR12-int/RetroMyelin sequences from multiple species to reconstruct its genetic tree. Apparently, the sequences within each species were grouped closely together, supporting the multi-event scenario.
Thus, RNLTR12-int/RetroMyelin integration likely happened independently in various species. We call this convergent evolution, where different species evolved similar traits on their own.
I also find it hard to believe this scenario is true. But convergent evolution does happen. Examples include the evolution of swimming-shaped bodies in fishes and mammals, the evolution of eyes with lenses in mammals and cephalopods (octopuses and squids), and the evolution of wing-like structures in birds, insects, and mammals (bats).
Why it Matters
But what exactly drove the evolution of the myelin sheath is unclear.
The co-emergence of myelin sheath and jaws may offer some hints. The development of jaws introduced a need for more refined neural control of the rapid movements involved in biting, chewing, and processing food. The faster nerve signaling— made possible by the myelin sheath —was likely vital in meeting these demands. Consequently, the evolution of both jaws and the myelin sheath may be a coordinated adaptation, enabling early vertebrates to become more efficient and complex predators.
That said, the retrovirus-mediated evolution of the myelin sheath is nothing short of marvelous. Without this pivotal evolutionary event, our nervous system wouldn’t reach its complexity today. Our neurological functions — our sensory, motor, and cognitive capabilities — would not be as efficient as they are without the myelin sheath.
“Retroviruses were required for vertebrate evolution to take off,” Prof. Robin Franklin, PhD, the study’s director, said. “If we didn’t have retroviruses sticking their sequences into the vertebrate genome, then myelination wouldn’t have happened, and without myelination, the whole diversity of vertebrates as we know it would never have happened.”
Such retrovirus-mediated evolution of the myelin sheath is a powerful demonstration of the monumental role of viruses in influencing our evolutionary history. In fact, 8% of our genomes are made of retroviral sequences called human endogenous retroviruses (HERVs). Moreover, another 40% of the human genome consists of retrotransposon sequences, which are also believed to have a viral origin.
One of these “non-human” genes also catalyzed the evolution of Arc, which is necessary for mediating the neurological processes that underpin learning and memory. I wrote about this in-depth here.
Ultimately, the story of viruses and the myelin sheath is a profound testament to the unpredictable nature of evolution. It reminds us of the intricate web of life, where even the smallest lifeforms can transform the course of evolution, where the most unexpected players can leave an enduring legacy, and where the convergence of seemingly unrelated forces can spark some of the most extraordinary innovations in nature.
This is how our neurons mastered electrical signals — thanks to a retrovirus infection hundreds of million years ago.
I may digress, but an interesting corollary to viruses and myelin sheath’s evolution is multiple sclerosis, a neurological disease by which the myelin sheath breaks down. Did you know that the causative agent of multiple sclerosis is actually proven to be another virus?
If you have made it this far, thank you. If you enjoyed this, please subscribe below and share it with others. You can also tip me here. :))





