The Reason Why Shingles Vaccine is Highly Protective Against Dementia
It may not have anything to do with shingles after all.
In the quest to battle dementia, the shingles vaccine may be more important than we think. Recent studies from the U.K., U.S., and Australia have reported fascinating cause-and-effect evidence that shingles vaccination could cut the incidence of dementia substantially, especially among older females.
In my previous article, “Shingles Vaccine Protects Against Dementia, But How Strong is the Effect? Here's My Attempt to Decode It,” I delved into the strength of this effect size and estimated the real-world impact of shingles vaccination on dementia rates based on these recent studies.
In this article, we’re exploring the "why" behind these findings. How could a vaccine aimed at preventing a skin condition provide a shield against dementia? To answer this, we need to understand the fascinating biology of shingles and the unique ways herpesviruses interact with the brain.
A Backdrop on Shingles
[Feel free to skip this section if you’re already familiar with shingles.]
Shingles begins when someone gets exposed to the varicella-zoster virus (VZV) through direct contact with an infected person or by inhaling virus particles in the air. Because of how contagious VZV is (R-naught of 5.7), most people get exposed to it during childhood and develop chickenpox.
Once infected, VZV doesn’t leave the body; instead, it lies dormant in nerve cells for life, typically in the nerve clusters along the spinal cord or brainstem. Scientists call this latency or a latent infection.
VZV can reactivate later in life, typically when the immune system weakens with age or is compromised, causing shingles. Also called herpes zoster, shingles is a painful skin rash that may come with fever, headache, and fatigue. Although it usually heals within 2-4 weeks, the rash can be severe (forming crusts and pus) in immunocompromised individuals.
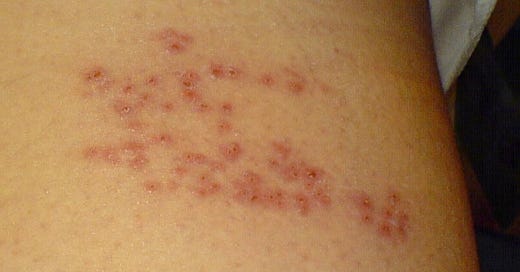
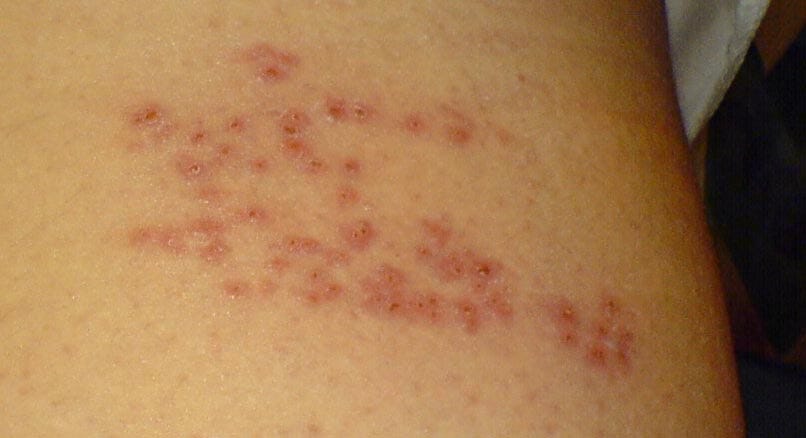
The site of shingles depends on where reactivated VZV infects.
If VZV reactivates near the spinal cord, it can cause shingles on one side of the torso, where the sensory nerves near the spinal cord are infected (Figure 1). In about 10-20% of cases, VZV can reactivate from the trigeminal ganglion in the brainstem and spread to the ophthalmic nerve, causing herpes zoster ophthalmicus (HZO) (Figure 2). As HZO can cause vision loss, it’s a medical emergency. In 5-10% of cases, VZV reactivation can cause chronic nerve pain lasting for months or years, known as post-herpetic neuralgia.


About 20% of chickenpox survivors will develop shingles later in life, usually after 50 years old. Each year, there are about 3-5 new cases of shingles per 1000 individuals on a global average. This incidence rises sharply after the age of 50 to 5-15 cases per 1000 individuals at 80 years (Figure 3).
Essentially, shingles is a neurological infection that manifests as a skin condition. Now, it makes sense why the shingles vaccine could protect against dementia, the most common neurodegenerative disease.
But how?

Hypothesis 1: Preventing Shingles (?)
Let’s start with the most obvious one: the shingles vaccine is designed to immunize against shingles, but it also confers protection against dementia. As follows, it makes sense to assume that shingles heighten the risk of dementia. By vaccinating against shingles, we also protect against dementia.
This hypothesis is substantiated by Taquet et al.’s landmark study in 2024. For context, this study capitalized on a naturally randomized setting to provide cause-and-effect evidence that Shingrix (recombinant shingles vaccine) was more effective than Zostavax (live shingles vaccine) in protecting against dementia. This finding builds on two previous similar studies by Eyting et al. and Pomirchy et al., which showed that Zostavax is already effective at cutting the incidence of dementia in a cause-and-effect manner.
I covered these three landmark studies in depth here.
In Taquet et al.’s study, the risk of shingles also decreased by 35% in those eligible for Shingrix than those eligible for Zostavax. Both types of vaccines prevent shingles, but the recombinant one (Shingrix) is recommended over the live version (Zostavax) due to better efficacy and safety. If shingles drives dementia, it helps explain why Shingrix better protects against dementia than Zostavax (because it’s more effective at preventing shingles).
But this hypothesis faces one problem: Taquet et al.’s study did not show that the reduction in shingles risk correlates with dementia risk.
In fact, a history of shingles doesn’t always lead to an increased risk of dementia. In a 2023 meta-analysis, Elhalag et al. found no significant difference in the incidence of dementia between people with and without shingles due to mixed results from various studies (Figure 4).
This means the two diseases, shingles and dementia, may not be related. It also means that the dementia-protective effects of the shingles vaccine are not simply because the vaccine prevents shingles.
Some other mechanisms must be at play.

Hypothesis 2: Preventing VZV Vascular Complications
Despite the null findings, Elhalag et al. actually found an association that was statistically significant: A staggering 6-fold increased risk of dementia among those who had herpes zoster ophthalmicus (HZO) (Figure 5).
Interestingly, patients with HZO also face a higher risk of stroke, per a 2017 meta-analysis. Specifically, the stroke risk increased by 2.1-fold at 3 months after HZO and remained elevated at 2.3-fold one year later (Figure 6). Similar findings were also reported recently in a 2023 cohort study in the U.S.
In line with this, the shingles vaccine has also been observed to reduce the risk of stroke, although more recent evidence suggests that this phenomenon could be due to the healthy vaccinee bias effect, where individuals who get vaccinated tend to be healthier overall. This bias can’t be controlled in typical observational studies, thus hindering cause-and-effect inference.
(But recent studies managed to overcome this bias to show that the shingles vaccine could reduce the risk of dementia — as I detailed in a previous article here — which forms the basis for the current article.)
And stroke is a strong risk factor for dementia. Stroke patients are estimated to face up to a 3-fold increased risk of dementia within the first year, from which the dementia risk gradually declines to about 1.5-2-fold.


Now, how is VZV related to stroke and then dementia?
Elhalag et al. hypothesized that:
VZV is the only human virus that is capable of replication in cerebral arteries and causes vasculopathy… Additionally, via the trigeminal nerve, namely from the ophthalmic branch of trigeminal afferent fibers, the virus travels trans-axonally to cerebral arteries, causing additional vascular inflammation and thrombosis that may subsequently damage brain cells.
To recapitulate, HZO occurs when reactivated varicella-zoster virus (VZV) spreads to and infects the ophthalmic nerve, i.e., the ocular division (V1) of the trigeminal nerve that originates from the brainstem (Figure 2).
From there, VZV can travel back to the brainstem since trans-axonal transport can be both anterograde (forward) and retrograde (backward). Alternatively, VZV can travel from the ophthalmic nerve to nearby cerebral arteries due to their close anatomical proximity (Figure 8).
As stated by Grose (2019):
[The] ophthalmic branch of the trigeminal ganglion also sends afferent branches along the cerebral arteries, including the middle cerebral artery, anterior cerebral artery and internal carotid artery. The terminals of the nerve fibers enter the adventitia surrounding the arteries. Thereafter the virus then can enter the arterial wall, to initiate an inflammatory process.

While the exact route of VZV infection of the cerebral arteries remains unclear, we know it can happen because of a clinical condition called VZV vasculopathy, i.e., inflamed cerebral arteries due to VZV infection.
Autopsy analysis of victims of VZV vasculopathy has revealed evidence of VZV particles in the cerebral arteries (Figure 9). In living patients with VZV vasculopathy, VZV genetic material can also be detected in the cerebrospinal fluid via polymerase chain reaction (PCR), and inflamed cerebral arteries can be seen via magnetic resonance imaging (MRI).
Therefore, a plausible mechanism by which the shingles vaccine protects against dementia is its ability to prevent vascular complications associated with VZV infection. And these vascular complications, such as inflammation and blood clots, are known contributors to dementia.

Hypothesis 3:
Keep reading with a 7-day free trial
Subscribe to The Infected Neuron to keep reading this post and get 7 days of free access to the full post archives.